Imagine walking into your chemistry lab, surrounded by bubbling beakers and colorful solutions. The air buzzes with anticipation, ready to unveil the secrets hidden within the world of chemistry. At the heart of this excitement lies the understanding of how atoms bind together to form molecules—the fundamental building blocks of our universe. This exploration takes us to two key types of bonds: ionic and covalent bonds. Understanding the properties of compounds formed through these bonds, and how they manifest during lab experiments, is crucial to grasping the essence of chemistry.
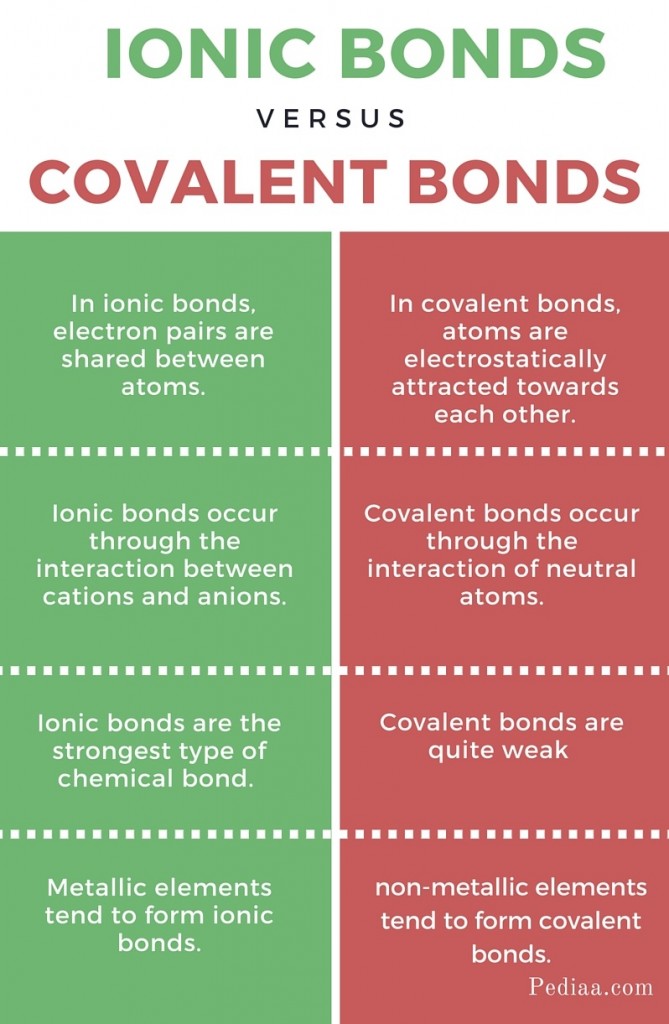
Image: www.aiophotoz.com
This article delves into the fascinating world of lab properties of ionic and covalent compounds. We’ll journey into the differences between these common bonds, unveil the distinct characteristics of their respective compounds, and explore how these properties come alive in hands-on lab experiments. Join us as we unlock the secrets behind these chemical interactions, fostering your understanding of the fundamental building blocks that shape the world around us.
The Dance of Atoms: A Closer Look at Ionic and Covalent Bonds
To understand the properties of ionic and covalent compounds, we need to start with the fundamental interactions driving their formation. These interactions are governed by the nature of chemical bonds.
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. Imagine two atoms, one with a strong desire to lose an electron and the other eager to gain one. This exchange of electrons leads to the formation of ions—atoms with a net positive or negative charge. The attraction between these oppositely charged particles creates the ionic bond. Think of it as a magnetic dance, drawing the ions together to form a stable compound.
Covalent bonds, on the other hand, involve the sharing of electrons between atoms. Like a shared birthday cake, atoms in covalent bonds contribute electrons to create a shared pair of electrons that both atoms feel a pull towards. This sharing of electrons creates a strong bond, as the atoms pull equally on the shared electrons, resulting in a stable, neutral molecule.
Unveiling Distinct Characteristics: A Comparative Study of Ionic and Covalent Compounds
The nature of the bonds within a compound dictates its properties. Let’s step into the laboratory and explore the distinct characteristics of ionic and covalent compounds.
1. Melting and Boiling Points: Ionic compounds typically have high melting and boiling points compared to covalent compounds. This is because the electrostatic forces holding the ions together are much stronger than the weaker forces holding covalent molecules together. Imagine trying to separate two magnets; you’ll need much more energy than separating two pieces of velcro.
2. Solubility: Ionic compounds are generally soluble in polar solvents like water. This is because the polar water molecules can interact with the charged ions, separating them and dissolving the compound. Covalent compounds tend to be soluble in non-polar solvents, like organic solvents.
3. Conductivity: Ionic compounds conduct electricity when dissolved in water or molten. This is because the free-moving ions can carry electric charge. Covalent compounds, on the other hand, generally do not conduct electricity in solution or as liquids. This is because they do not form free-moving ions.
4. State at Room Temperature: Ionic compounds are often solids at room temperature due to the strong electrostatic forces between ions. Covalent compounds can exist as solids, liquids, or gases at room temperature depending on the strength of the intermolecular forces between the molecules.
Hands-on Exploration: Bringing Lab Properties to Life
Now, let’s bring these concepts to life through classic lab experiments.
-
Experiment 1: Melting Point Determination: By carefully heating ionic and covalent compounds, we can observe their distinct melting points. This experiment visually reinforces the concept of strong ionic forces versus weaker covalent forces.
-
Experiment 2: Conductivity Test: Using a conductivity apparatus, we can test the conductivity of different solutions. The experiment will show that ionic compounds conduct electricity when dissolved but covalent compounds do not.
-
Experiment 3: Solubility Observations: By observing the solubility of different compounds in water, we can further illustrate the polar-nonpolar interactions involved.
These experiments provide a unique hands-on experience to observe and analyze the fascinating properties of ionic and covalent compounds. You’ll gain firsthand experience witnessing the distinct characteristics of these fundamental chemical bonds.

Image: www.studypool.com
Unlocking the Secrets of Your World: Practical Applications of Bonding Concepts
Understanding the properties of ionic and covalent compounds is essential for unlocking the secrets of the world around us. From the everyday things we use to advanced technologies, these principles are woven into the fabric of our modern world.
-
Medicine: Many medications are designed using ionic compounds due to their ability to dissolve in body fluids and deliver therapeutic effects.
-
Agriculture: Fertilizers rely heavily on ionic compounds, supplying essential nutrients to plants.
-
Clean Energy: Solar panels utilize the properties of semiconductors, often made of covalent compounds, to convert sunlight into electricity.
-
Materials Science: The properties of ionic and covalent compounds are essential for developing new materials, from strong plastics to high-performance ceramics.
By understanding the properties of ionic and covalent compounds, we unlock the potential to develop new technologies, solve important problems, and create a better future.
Lab Properties Of Ionic And Covalent Compounds Answer Key Pdf
Conclusion: Embark on Your Chemical Journey
Understanding the world of chemical bonding is a journey of discovery. As we delve deeper into the properties of ionic and covalent compounds, we reveal the secrets of the building blocks that shape our world. This knowledge empowers us to harness the power of chemistry, from developing new materials to understanding the complexities of our bodies.
So, embark on your own chemical journey! Explore the wonders of ionic and covalent bonds through hands-on experiments, engage with the scientific literature, and apply your newfound knowledge to solve real-world problems. Together, let’s unlock the exciting world of chemistry and its boundless potential to shape our future.






