Ever wondered how many kilograms a liter of water weighs? It’s a question that pops up in various contexts, from cooking to grocery shopping. I remember struggling with this very question a few years back while trying to convert a recipe from liters to kilograms. It seemed like a simple conversion, but the answer eluded me. That day, I realized the importance of understanding volume and mass, especially when dealing with liquids.
Image: judahfersroberts.blogspot.com
This article explores the relationship between liters (L) and kilograms (kg), particularly in the context of water. We’ll delve into the science behind the conversion, unravel the complications, and guide you on how to convert between these units.
Understanding Liters vs. Kilograms
Liters and kilograms measure different aspects of a substance:
- Liter (L): A unit of volume, indicating the space a substance occupies.
- Kilogram (kg): A unit of mass, indicating the amount of matter a substance contains.
Imagine two containers: one filled with feathers and the other with rocks. Both containers could occupy the same volume (1 liter), but the container with rocks will be much heavier due to its greater mass. While volume is the space an object takes up, mass is the amount of matter within that space.
The Importance of Density
Converting liters to kilograms requires understanding the concept of density. Density is a fundamental property of any material, defined as its mass per unit volume. The formula is:
Density = Mass / Volume
The density of a substance tells you how tightly packed its particles are. A high-density substance has more mass packed into a smaller volume. For example, iron is denser than water because a small lump of iron has more mass than an equal volume of water.
How Density Applies to Water
The key to converting liters to kilograms is realizing that the density of water is approximately 1 kilogram per liter (1 kg/L) at 4°C. This means that 1 liter of water weighs roughly 1 kilogram.
However, this conversion holds true only for pure water at standard conditions. The density of water can fluctuate based on:
- Temperature: Density decreases as water warms. Hot water is slightly less dense than cold water.
- Pressure: Pressure affects density to a lesser extent. Increased pressure squeezes water molecules closer, increasing density. However, this effect is typically negligible in everyday situations.
- Dissolved Substances: Adding salt or sugar to water increases its density.
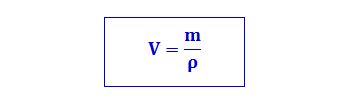
Image: quora.co.id
Converting Liters to Kilograms: A Step-by-Step Guide
If you’re dealing with pure water at standard temperature and pressure (4°C), you can use the following steps:
- Identify the volume in liters (L).
- Multiply the volume by the density of water (1 kg/L).
- The result is the mass in kilograms (kg).
For example, converting 2 liters of water to kilograms:
2 L × 1 kg/L = 2 kg
Therefore, 2 liters of water weighs approximately 2 kilograms.
Beyond Water: Converting Liter to Kilogram for Other Liquids
Converting liters to kilograms for liquids other than water requires knowing their specific density. You can find the density of various liquids online or in chemistry textbooks. Once you know the density of a liquid, you can use the same equation:
Mass (kg) = Volume (L) × Density (kg/L)
For instance, let’s say you want to convert 0.5 liters of olive oil into kilograms. The density of olive oil is roughly 0.91 kg/L. Applying the formula:
Mass (kg) = 0.5 L × 0.91 kg/L = 0.455 kg
Therefore, 0.5 liters of olive oil weighs approximately 0.455 kilograms.
Tips and Expert Advice for Accurate Conversions
Here are some tips for accurate conversion:
- Check the density: Ensure you are using the correct density for the specific liquid you are dealing with, considering temperature and dissolved substances.
- Use reliable sources: Consult reputable websites or textbooks for accurate density values.
- Consider temperature: If the temperature of your liquid deviates significantly from 4°C, adjust your density value accordingly. Check online resources for temperature-specific density data.
Remember, the density of liquids can fluctuate slightly, so using these conversion methods yields approximate values. Always consult density tables for precise calculations, particularly in scientific contexts.
Frequently Asked Questions
Here are answers to common questions:
Q: Can I apply the 1 liter = 1 kg conversion to all liquids?
A: No, only pure water at 4°C has a density of 1 kg/L. Other liquids have different densities, so you need to consider the specific density for accurate conversions.
Q: Why are liters and kilograms different units?
A: Liters measure volume, the space occupied by an object. Kilograms measure mass, the amount of matter in an object. Think of a balloon filled with air vs. a tiny piece of lead. The balloon could have a larger volume but much less mass than the small piece of lead.
Q: How important is it to understand the difference between liters and kilograms?
A: Understanding the difference is crucial for accurate conversions, especially when dealing with recipes, scientific experiments, or situations involving different densities.
1 Litre Is How Many Kg
Conclusion
Converting liters to kilograms involves understanding density – the key link between volume and mass. For pure water at 4°C, 1 liter directly equates to 1 kilogram. However, for other liquids, you need to consider their specific densities for accurate conversion. By applying the correct formulas and utilizing reliable sources for density values, you can confidently convert between these units.
Are you now confident in converting liters to kilograms? Let us know in the comments below!






